presents
Oceanography
Water, Seawater and Ocean Circulation and Dynamics
by
Jason
Buchheim
Director, Odyssey Expeditions
presents
Oceanography
Water, Seawater and Ocean Circulation and Dynamics
by
Jason
Buchheim
Director, Odyssey Expeditions
The oceans, the big blue, source of life, the hallmark of Earth. We hold the oceans within us, both physically and mentally. Vast, blue, tranquil, and treacherous, the oceans are the signature of our planet. The only planet in the solar system blessed with a liquid medium for life to evolve in.
The motions of the atmosphere, traced out by clouds, and the size of the oceans dominate the view of earth from space. So vast are the oceans, in fact, that they take up almost 71% of the entire surface of the globe (139 million square miles). The oceans have an average depth of 12,230 feet (3730 m) and reach the deepest point in the Mariana Trench of the northwester Pacific Ocean, at 36,204 feet (11,038m) below sea level. The ocean basins hold at vast quantity of water, over 285 million cubic miles of water (1185 million cu. km.). This vast quantity of water arose from the Earth's interior as it cooled.
The oceans are the largest repository of organisms on the planet, with representatives from all phylum's. Life is extremely abundant in the sea, from the obvious large whales, fish, corals, shrimp, krill and seaweed, to the microscopic bacteria floating freely in the seas. The bacteria is so abundant that just one spoonful of ocean water contains from 100 - 1,000,000 bacteria cells per cubic centimeter!
The oceans contain the largest repository of organisms on the planet, and all the organisms in the ocean are subject to the properties of the seawater surrounding them.
Water surrounds all marine organisms, composes the greater bulk of their bodies, and is the medium by which various chemical reactions take place, both inside and outside of their bodies.
In this chapter, we present the basic chemistry of water, a necessary step in understanding the interesting roles water plays as an extremely suitable medium for life.
| Water itself is very simple. Each molecule of water is composed of two hydrogen atoms and one oxygen atom. The hydrogen atoms bond to the oxygen atom asymmetrically by sharing electrons (Each hydrogen atoms shares its only electron with the oxygen atom. The oxygen atoms receives the two electrons needed to complete its outer shell, making it a stable molecule.) | 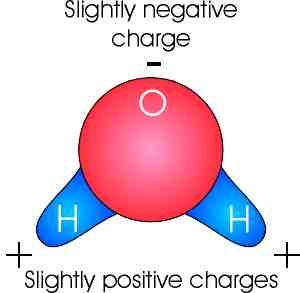 |
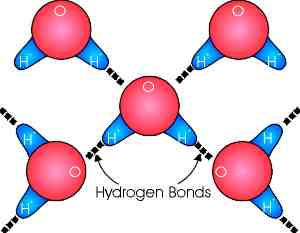 |
Important interactions occur because of the electron sharing. The oxygen atoms tends to draw the electrons furnished by the Hydrogen atoms closer to its nucleus, creating an electrical separation and a polar molecule. The polar nature results in the hydrogen end (which ahs a positive charge) attracting the oxygen end (with a negative charge) of other adjacent water molecules. This forms Hydrogen Bonds between adjacent water molecules. These bonds are weak compared to the electron sharing bonds (6% as strong) and are easily broken and reformed. |
The hydrogen bonding and polarity of water molecules is responsible for many of the unique characteristics and physical properties of water.
Seawater
Seawater is pure water plus dissolved solids and gases. The dissolved solids come from 'weathering' processes of the continental land masses rocks being dissolved by rain water and flowing out to sea with the rivers. The gases come from the atmosphere. As water is a universal solvent, many different compounds are dissolved in it. A 1kg sample of saltwater contains 35 g of dissolved compounds, including inorganic salts, organic compounds from living organisms, and dissolved gasses. The solid substances are known as 'salts' and their total amount in the water is referred to by a term known as Salinity (expressed as parts per thousand). Oceanic salinities generally range from 34 to 37 parts per thousand. Variations from place to place are due to factors such as rainfall, evaporation, biological activity and radioactive decay. Salinities are higher in the tropics due to high evaporation rates. Fresh supplies of salts are now being added to the oceans from the rivers at roughly the same rate that they are being removed by various physical, chemical and biological processes.
| Inorganic salts compose most of the solid
matter of the 'salts' (99.28%)
These percentages remain constant regardless of the waters salinity; therefore, salinity can be measured by measuring just the concentration of one of the salts, such as chlorine. The remaining .72% of the 'salts' are inorganic salts crucial to life. These include phosphates, nitrates, (both nutrients required for photosynthesis) and silicon dioxide (required by diatoms to construct their glass skeletons). In contrast to the other salts, the nitrates and phosphates vary in concentration due to biological activity. In surface waters, where plants are actively in the process of photosynthesis, the nitrates and phosphates can be in short supply, limiting the amount of biological activity that can take place. |
|
Temperature
Temperature is a very important physical parameter in the marine environment. It limits the distribution and ranges of ocean life by affecting the density, salinity, and concentration of dissolved gasses in the oceans, as well as influencing the metabolic rates and reproductive cycles of marine organisms.
The seasonal range of temperature in the ocean is affected by latitude , depth, and proximity to the shore. Marine temperatures change gradually because of the heat capacity of water. In the abyssal zone, water temperatures are remarkably stable and remain virtually constant throughout the year. Similarly, in equatorial and polar marine regions, ocean temperatures change very little with season.
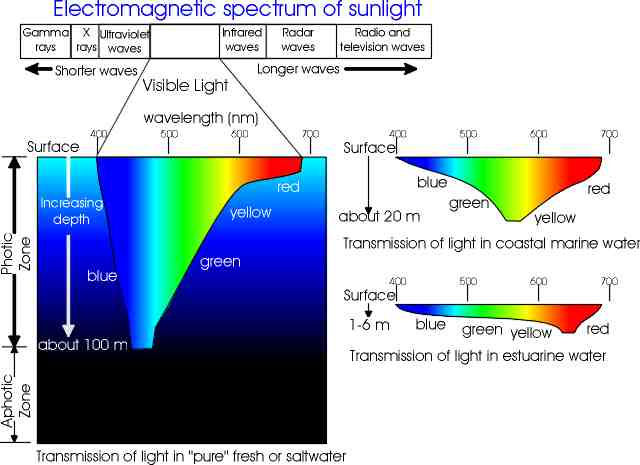
Because the surface of the ocean is heated by sunlight, the depths are cooler. There is a minimum of vertical mixing, because the warm water cannot displace the dense, colder deep water.
December 1995 Temperature Anomalies
The waters of the ocean are in constant motion. Its movement ranges from strong currents such as the Gulf Stream, down to small swirls or eddies.
What causes all of this motion?
The short answer is: energy from the Sun, and the rotation of the Earth.
The Sun drives oceanic circulation in two primary ways:
The rotation of the Earth contributes to ocean circulation patterns:
Consider the following: A missile fired due northwards from a launch pad at the equator.
however, as the eastward velocity at the surface of the earth is greatest
at the Equator and decreases towards the poles, as the missile travels
north, the eastward velocity of the Earth below the missile becomes less
and less.
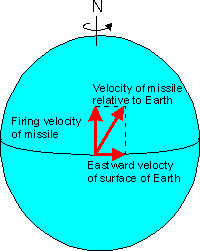 |
A missile
launched from the Equator has both its northerly firing velocity and an
eastward velocity relative to the surface of the Earth at the equator.
Its actual relative travel follows a resultant vector which is a combination of the two. |
| The path taken by a missile
has a deflection attributed to the Coriolis force. The coriolis force
increases with increasing latitude.
The blue paths shown indicate the courses taken by a missile or any other body moving over the surface of the Earth without being strongly bound by friction. |
Because a missile is moving so fast, the amount that the Earth has 'turned beneath" it during its short flight is small. Winds and ocean currents, on the other hand, are slow moving, and so are significantly affected by the Coriolis force.
The Coriolis force therefore has a significant effect on deflecting
ocean currents. 
Marine Biology resources by Odyssey Expeditions Tropical Marine Biology Voyages | ||
|